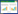2 徐州医科大学附属连云港医院, 连云港市第一人民医院影像科, 222000;
3 徐州医科大学附属连云港医院, 连云港市第一人民医院急诊医学科, 222000
2 Department of Radiology, Lianyungang Hospital Affiliated to Xuzhou Medical University, Lianyungang, 222000 China;
3 Department of Emergency Medicine, Lianyungang Hospital Affiliated to Xuzhou Medical University, Lianyungang, 222000 China
ICU获得性肌无力(ICU-acquired weakness, ICU-AW)是重症患者出现脱机困难、全身无力或四肢瘫痪、各种反射减弱甚至消失及肌肉萎缩等临床表现,延长患者住院时间,导致患者长期功能障碍,增加病死率,严重影响患者预后[1-2]。ICU机械通气患者ICU-AW发生率高达60%,对ICU-AW的早期诊断和及时干预至关重要。但是目前尚无对ICU-AW评估和诊断的“金标准”[3],尤其缺乏具有诊断价值的生物标志物。生长分化因子-15(growth differentiation factor-15, GDF-15)是一种转化生长因子β蛋白,已被证实参与COPD、癌症及肺动脉高压等多种疾病的肌肉萎缩的发展,与骨骼肌肌肉消耗有关[4-6]。近期国外一些研究表明[7-8],竖脊肌横截面积可以作为患者肌肉含量的评估方法。本研究是一项观察性研究,旨在明确竖脊肌横截面积及GDF-15与ICU机械通气患者的急性肌肉消耗之间的联系,并评估其用于诊断ICU获得性肌无力的效用,及对ICU机械通气患者60 d生存状态预测价值,以便及时制定临床干预措施。
1 资料与方法 1.1 一般资料依次筛选2018年6月至2019年11月本院ICU病房的需要有创机械通气治疗的神志清楚的急性呼吸衰竭患者。
1.1.1 纳入标准年龄≥18周岁;需要机械通气治疗,意识状态水平能够配合完成简单肌力测定,预估机械通气治疗超过5 d;ICU住院时间≥7 d。
1.1.2 排除标准神志不清,不能配合检查;颅脑外伤、脊髓损伤、脑卒中急性期;急性周围神经肌肉病变如格林巴利综合征、周围神经炎等;肢体严重骨折;既往有中枢性认知功能障碍或神经肌肉疾病;需要肌松药物治疗者;存在急慢性心功能不全及急性心肌梗死患者。
1.1.3 剔除标准自动出院或发生死亡致住院时间少于7 d者,住院过程中并发神经系统疾病,不能完成胸部CT复查者。
1.2 伦理审查及临床试验注册本研究符合医学伦理学标准,经医院伦理委员会批准,审批号为LCYJ20171228001,所有患者入组均取得患者家属同意并签署知情同意书。中国临床试验中心注册号为ChiCTR1900025382。
1.3 研究方法 1.3.1 胸部CT扫描和竖脊肌横截面积测量分析所有纳入患者在入院前或入院首日即行胸部CT扫描检查,并于治疗7日后复查胸部CT,在胸部CT应用纵隔窗进行竖脊肌横截面积分析。定位T12椎体下缘水平分析竖脊肌[9]。详细记录竖脊肌横断面面积测量结果,在CT图像上识别左右竖脊肌,并手动勾画竖脊肌伪彩,计算两边竖脊肌横断面积和脊柱左右竖脊肌总的横断面积,见图 1。

|
| 图 1 同一患者竖脊肌横截面积测量及对比 Fig 1 Measurement and comparison of cross-sectional area of erector spinae in the same patient |
|
|
分别在患者入住ICU的第1天及第7天,用抗凝管采集肘正中静脉血3 mL,经3 000 r/min速度离心5 min,用1 mL离心管收集上清液并保存于-80℃中备用。采用酶联免疫吸附法对研究对象血清GDF-15浓度水平进行检测。检测试剂盒购于美国Life-tech公司,实验步骤按照说明书步骤进行操作。
1.3.3 肌肉力量评定在患者入住ICU的第1天及第7天停用所有镇静及镇痛药物,保持患者意识清醒状态,RASS评分0至+1分,能够有效配合检查,采用英国医学研究委员会(the British Medical Research Council, MRC)肌力评定法对患者躯体六大肌群(双侧腕伸展、前臂屈曲、肩外展、足背屈、膝伸展、大腿弯曲)进行评估,每组肌群的肌力按牛津肌力等级评分,总分60分,得分<48分诊断为ICU获得性肌无力。肌力分级评分由两位经过培训的医师同时完成。
1.4 统计学方法采用Graphpad Prism6.0及SPSS22.0统计学软件进行数据处理及作图。计量资料用均数±标准差(Mean±SD)表示,两组间差异比较使用两独立样本t检验,计数资料比较采用χ2检验。根据患者入ICU第7天MRC-score评分将患者分为ICU-AW组和非ICU-AW组,比较并分析两组患者分别在入ICU第1天及第7天ESMcsa、GDF-15及MRC-score差异。采用Pearson相关性分析对所有患者第7天ESMcsa Loss、GDF-15及MRC-score进行相关性分析。采用受试者工作特征曲线(ROC)分别计算患者第7天ESMcsa Loss、ESMcsa Loss Ratio及GDF-15水平对机械通气患者ICU-AW诊断效能。绘制患者Kaplan-Meier生存曲线,分析ESMcsa Loss Ratio及GDF-15对患者60 d生存状态预测价值。以P<0.05为差异有统计学意义。
2 结果 2.1 一般临床资料的比较筛选患者153例,其中42例因不符合纳入标准,符合排除标准,排除入组,累计入组111列,因自动出院或早期发生死亡致治疗时间少于7 d剔除入组12例,因各种原因致7 d后未行胸部CT检查,无法完成ESMcsa测量剔除7例,最终纳入统计患者92例,其中男性61例,女性31例。记录统计患者基线水平资料,并进行SOFA评分及24 h APACHEⅡ评分,统计患者机械通气治疗时间,ICU住院时间及医院住院时间。
ICU-AW组与非ICU-AW组在性别、年龄、体质量、体质指数、基础病史、吸烟史、机械通气病因、急性生理和慢性健康评分(APACHEⅡ评分)及序惯性器官衰竭评分(SOFA评分)等基线水平比较,差异无统计学意义(均P>0.05);两组机械通气治疗时间,ICU住院时间及医院住院时间比较,差异有统计学意义(均P<0.05),见表 1。
| 指标/组别 | ICU-AW组 (n=49) |
非ICU-AW组 (n=43) |
t/χ2值 | P值 |
| 年龄(岁) | 61.34±12.41 | 60.53±11.36 | 0.325 | 0.746 |
| 女/男 | 17/32 | 14/29 | 0.216 | 0.829 |
| 体质量(Kg) | 62.38 ±8.42 | 63.74±10.63 | 0.684 | 0.496 |
| BMI(Kg/m2) | 24.53±3.52 | 25.72±3.84 | 1.551 | 0.125 |
| SOFA评分 | 8.92±3.52 | 8.79±3.69 | 0.173 | 0.863 |
| APACHEⅡ | 17.29±7.31 | 16.57±8.24 | 0.444 | 0.658 |
| 吸烟史 | 17/49 | 13/43 | 0.455 | 0.649 |
| 基础病史 | 21/49 | 17/43 | 0.323 | 0.747 |
| 机械通气病因 | ||||
| 肺部感染 | 14/49 | 15/43 | 0.650 | 0.516 |
| AECOPD | 16/49 | 13/43 | 0.249 | 0.803 |
| 肺挫伤 | 6/49 | 5/43 | 0.091 | 0.927 |
| ARDS | 7/49 | 5/43 | 0.378 | 0.706 |
| 其他 | 6/49 | 7/43 | 0.554 | 0.579 |
| 机械通气时间(h) | 189.87±53.43 | 163.47±48.81 | 2.462 | 0.015 |
| ICU住院时间(d) | 11.76±3.42 | 9.53±2.67 | 3.451 | 0.001 |
| 医院住院时间(d) | 19.23±6.57 | 16.45±4.76 | 2.295 | 0.024 |
| 注:基础病史包括高血压、糖尿病、慢性肾病、外伤手术史等 | ||||
两组间比较发现,患者ESMcsa、血清GDF-15、MRC-score评分在第1天差异无统计学意义(均P>0.05),而ICU-AW组在第7天的血清GDF-15明显高于非ICU-AW组,ESMcsa及MRC-score评分明显低于非ICU-AW组,差异有统计学意义(均P<0.05),见表 2。ICU-AW组第7天ESMcsa较第1天自身对照比较显著减小,提示存在急性肌肉消耗致骨骼肌含量显著降低,而非ICU-AW组肌肉含量损耗并不显著,见图 2。
| 组别 | 第1天 | 第7天 |
| ESMcsa(cm2) | ||
| ICU-AW组(n=49) | 29.52±8.32 | 23.76±6.85 |
| 非ICU-AW组(n=43) | 30.16±6.74 | 29.15±6.51 |
| t值 | 0.40 | 3.85 |
| P值 | 0.69 | 0.00 |
| GDF-15(pg/mL) | ||
| ICU-AW组(n=49) | 1211.24±338.23 | 2529.53±625.67 |
| 非ICU-AW组(n=43) | 1095.29±274.22 | 1614.21±567.18 |
| t值 | 1.82 | 7.31 |
| P值 | 0.07 | 0.00 |
| MRC-score | ||
| ICU-AW组(n=49) | 55.00±2.62 | 41.10±3.35 |
| 非ICU-AW组(n=43) | 54.51±2.75 | 51.23±2.84 |
| t值 | 0.87 | 17.03 |
| P值 | 0.39 | 0.00 |

|
| 图 2 两组患者7 d内竖脊肌横截面积变化趋势图 Fig 2 The trend of the cross-sectional area of the erector spinae in the two groups within 7 days |
|
|
Pearson相关性分析显示,ESMcsa Loss及ESMcsa Loss Ratio与第7天血清GDF-15水平呈显著正相关(r分别0.2355和0.3192,均P<0.01,见图 3A/C),ESMcsa Loss及ESMcsa Loss Ratio与MRC-score评分呈显著负相关(r分别-0.3072和-0.3527,均P<0.001,见图 3B/D)。

|
| 图 3 患者ESMcsa Loss、ESMcsa Loss Ratio与第7天血清GDF-15浓度及MRC-score相关性分析 Fig 3 Correlation analysis of ESMcsa loss and ESMcsa loss ratio with serum GDF-15 concentration and MRC-score on day 7 |
|
|
采用受试者工作特征曲线(ROC)分析患者第7天ESMcsa Loss、ESMcsa Loss Ratio及第7天血清GDF-15水平对ICU-AW诊断的预测价值显示:第7天ESMcsa Loss曲线下面积为0.835,最佳截断值为4.20 cm2时,敏感性88.9%,特异性为68.0%;ESMcsa Loss Ratio曲线下面积为0.889,最佳截断值为13.5%时,敏感性91.1%,特异性为76.0%,血清GDF-15水平曲线下面积(AUC)0.904,最佳截断值为2 214 pg/mL时,敏感性91.2%,特异性为76.6%;见图 4。

|
| 图 4 ESMcsa Loss、ESMcsa Loss Ratio及血清GDF-15诊断预测ICU-AW的ROC曲线 Fig 4 ROC curve of ESMcsa loss, ESMcsa loss ratio and GDF-15 for ICU-AW diagnosis |
|
|
统计所有入组患者60 d生存率情况,根据ROC曲线对第7天ESMcsa Loss Ratio的最佳截断值取值水平,将所有入组患者分为高ESMcsa Loss Ratio组和低ESMcsa Loss Ratio组,绘制患者生存曲线图,比较两组患者60 d生存率,结果显示高ESMcsa Loss Ratio组生存率为60.0%,低ESMcsa Loss Ratio组生存率为80.0%,两组60 d生存率差异有显著统计学意义(P<0.05),根据ROC曲线对第7天GDF-15血清浓度的最佳截断值取值水平,将所有入组患者分为高血清GDF-15浓度组和低血清GDF-15浓度组,绘制患者生存曲线图,比较两组患者60 d生存率,结果显示高GDF-15浓度组生存率为60.0%,低GDF-15浓度组生存率为77.8%,两组在60 d生存率差异有显著统计学意义(P<0.05),见图 5。

|
| 图 5 ESMcsa Loss Ratio及GDF-15水平Kaplan-Meier生存曲线 Fig 5 Kaplan-Meier survival curve of ESMcsa loss ratio and GDF-15 levels |
|
|
ICU-AW在重症患者中的发生率较高,常因患者意识障碍或使用镇痛镇静药物等导致病情掩盖,从而延误诊断和治疗[9]。寻找客观指标及生物标志物对其早期诊断和评估非常重要。
本研究发现,ICU-AW组机械通气时间,ICU住院时间及医院住院时间均显著高于非ICU-AW组,这与既往研究相符合[10],说明ICU-AW患者容易合并呼吸肌无力,导致脱机时间延长,可使危重病患者病程更复杂,影响患者预后[11]。随治疗时间的延长,ICU-AW组MRC-score评分逐渐降低,而血清GDF-15呈明显上升趋势,至第7日GDF-15浓度明显高于非ICU-AW组,同时CT影像学发现患者的竖脊肌横截面积较前显著减少,提示存在明显的肌肉消耗[11]。在ICU-AW的发病机制中,肌肉蛋白代谢紊乱起到重要作用[14]。分解代谢旺盛是危重症患者特别是老年患者重要的代谢特征,肌肉蛋白分解是其重要的组成部分,直接促进ICU-AW的发生[14]。目前认为肌肉蛋白降解主要是通过泛素-蛋白酶体和自噬-溶酶体等途径进行。当蛋白降解途径异常激活时, 蛋白降解加速致肌肉蛋白减少,导致肌肉萎缩[15]。而细胞因子GDF-15作为蛋白合成的重要调节因子之一[15],当其在体内异常出现时, 可减少肌肉蛋白合成, 导致肌肉萎缩。Bloch等[15]发现入住ICU的心脏术后高危患者GDF-15长期升高,而后进行的体外试验也证实GDF-15可引起肌管萎缩。并进一步从ICU-AW患者股直肌活检肌肉组织的研究中证明GDF-15可能通过增加TGF-β信号的敏感性来抑制肌肉miRNA表达, 从而促进了肌萎缩的发生。
目前诊断ICU-AW较为一致的观点仍然是应用MRC-Score评分对六大肌群进行评估,以总分<48分作为ICU-AW的诊断标准[11],但意识障碍及ICU镇静治疗严重的限制了其使用范围。本研究发现竖脊肌横截面积减少量及减少率对ICU-AW具有一定的临床诊断价值,AUC分别为0.835和0.889,说明它们均可以作为客观指标用于ICU-AW的临床诊断。在ICU-AW的发病机制中,骨骼肌质量急性减少是导致患者肌肉力量减退的重要原因之一,由于竖脊肌是人体质量要的抗重力骨骼肌之一,临床研究发现ESMcsa可以客观反映患者骨骼肌肉含量及功能[19],并与COPD及肺纤维化等肺部疾病预后相关[19]。它可以综合参数反映了肺部疾病患者体力活动和生理严重程度的指标,是COPD患者死亡的强烈危险因素之一[8]。本研究发现ICU住院患者因急性消耗、营养不足及卧床容易出现急性肌肉消耗,导致骨骼肌萎缩,肌肉功能及力量急性减退。对于机械通气患者采用竖脊肌横截面积作为评价指标,相比其他骨骼肌如胸大肌、大腿中部股四头肌、腰大肌等,胸12椎体ESMcsa的测量具有更多优势。它不受上下肢位置体位影响,在行胸部CT检查同时可以获得该参数指标,无需其他扫描和X射线暴露。此外在本项研究中,发现患者竖脊肌横截面积丢失、竖脊肌横截面积丢失率与第7天血清GDF-15水平呈显著正相关,说明GDF-15作为反映肌肉损耗的生物标志物与影像学客观测定竖脊肌肌肉质量具有很好的内在联系,能够协同评估患者肌肉损耗程度。它们与反映患者肌肉功能的MRC-score评分也同时具有一定的相关性,各参数指标之间可以互补协同,尤其对于ICU昏迷或者镇静治疗患者无法行MRC-score肌力评估时,能够在早期诊断及评估患者是否合并ICU获得性肌无力。
同时本研究发现,患者竖脊肌横截面积短时间内出现较大丢失量与高血清GDF-15浓度水平均可提示患者60 d可能存在更差的预后,这可能与临床上患者合并ICU-AW后病死率升高相一致。这说明急性肌肉消耗能够严重降低危重症患者的生存质量,影响患者预后,原因考虑与患者系统性器官功能不全,营养代谢失衡,营养不良,疾病消耗增加,运动量急剧减少相关。同时对于急性呼吸衰竭患者,呼吸肌无力使患者机械通气时间延长,发生呼吸机相关性肺炎等并发症几率增加,气管插管拔管失败率增加[20],肢体无力增加患者卧床时间及卧床并发症等因素有关[21]。
本研究是一项观察性研究,只能提示GDF-15与ICU获得性肌无力患者的肌肉丢失相关,并不能证明GDF-15与肌肉丢失之间存在因果关系。本研究并没有对ICU获得性肌无力肌肉萎缩的机制进一步探索。此外,本研究为单中心的小样本试验,需要进一步的多中心的大样本的临床研究确认ESMcsa和GDF-15在评价ICU获得性肌无力上的价值。
ICU机械通气患者竖脊肌横截面积丢失与血清GDF-15浓度的持续性升高呈显著相关,均可提示骨骼肌肌肉消耗及功能减退,是诊断ICU-AW的较好客观指标。并且竖脊肌横截面积急性的丢失与持续性升高的血清GDF-15浓度提示患者可能存在更差的预后,对预测ICU机械通气患者60 d生存状态有一定的临床意义。
利益冲突 所有作者均声明不存在利益冲突
| [1] | Patejdl R, Walter U, Rosener S, et al. Muscular Ultrasound, Syndecan-1 and Procalcitonin Serum Levels to Assess Intensive Care Unit-Acquired Weakness[J]. Can J Neurol Sci, 2019, 46(2): 234-242. DOI:10.1017/cjn.2018.390 |
| [2] | Bissett B M, Leditschke I A, Neeman T, et al. Inspiratory muscle training to enhance recovery from mechanical ventilation: a randomised trial[J]. Thorax, 2016, 71(9): 812-819. DOI:10.1136/thoraxjnl-2016-208279 |
| [3] | 王海旭, 张晓娟, 段晓光, 等. ICU获得性肌无力的诊断与治疗[J]. 中华急诊医学杂志, 2017, 26(10): 1187-1190. DOI:10.3760/cma.j.issn.1671-0282.2017.10.019 |
| [4] | Desmedt S, Desmedt V, de Vos L, et al. Growth differentiation factor 15: a novel biomarker with high clinical potential[J]. Crit Rev Clin Lab Sci, 2019, 56(5): 333-350. DOI:10.1080/10408363.2019.1615034 |
| [5] | Miller J, Skipworth RJE. Novel molecular targets of muscle wasting in cancer patients[J]. Curr Opin Clin Nutr Metab Care, 2019, 22(3): 196-204. DOI:10.1097/mco.0000000000000555 |
| [6] | Patel MS, Lee J, Baz M, et al. Growth differentiation factor-15 is associated with muscle mass in chronic obstructive pulmonary disease and promotes muscle wastingin vivo[J]. J Cachexia Sarcopenia Muscle, 2016, 7(4): 436-448. DOI:10.1002/jcsm.12096 |
| [7] | Tanimura K, Sato S, Fuseya Y, et al. Quantitative assessment of erector spinae muscles in patients with chronic obstructive pulmonary disease. Novel chest computed tomography-derived index for prognosis[J]. Annals ATS, 2016, 13(3): 334-341. DOI:10.1513/annalsats.201507-446oc |
| [8] | Asakura T, Yamada Y, Suzuki S, et al. Quantitative assessment of erector spinae muscles in patients with Mycobacterium avium complex lung disease[J]. Respir Med, 2018, 145: 66-72. DOI:10.1016/j.rmed.2018.10.023 |
| [9] | Veldema J, Bosl K, Kugler P, et al. Cycle ergometer training vs resistance training in ICU-acquired weakness[J]. Acta Neurol Scand, 2019, 140(1): 62-71. DOI:10.1111/ane.13102 |
| [10] | Dres M, Dubé BP, Mayaux J, et al. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients[J]. Am J Respir Crit Care Med, 2017, 195(1): 57-66. DOI:10.1164/rccm.201602-0367oc |
| [11] | Xing XZ. Effect of sedation on short-term and long-term outcomes of critically ill patients with acute respiratory insufficiency[J]. World J Emerg Med, 2015, 6(2): 147. DOI:10.5847/wjem.j.1920-8642.2015.02.011 |
| [12] | Borges RC, Soriano FG. Association between muscle wasting and muscle strength in patients who developed severe Sepsis and septic shock[J]. Shock, 2019, 51(3): 312-320. DOI:10.1097/shk.0000000000001183 |
| [13] | Petrof B J. Diaphragm Weakness in the Critically Ill: Basic Mechanisms Reveal Therapeutic Opportunities[J]. Chest, 2018, 154(6): 1395-1403. DOI:10.1016/j.chest.2018.08.1028 |
| [14] | Witteveen E, Wieske L, Sommers J, et al. Early prediction of intensive care unit-acquired weakness: a multicenter external validation study[J]. J Intensive Care Med, 2020, 35(6): 595-605. DOI:10.1177/0885066618771001 |
| [15] | 孟繁甦, 苏磊, 唐柚青, 等. 泛素蛋白酶体在骨骼肌高分解代谢中的意义[J]. 中华急诊医学杂志, 2009, 18(2): 216-218. DOI:10.3760/cma.j.issn.1671-0282.2009.02.030 |
| [16] | Hofmann M, Schober-Halper B, Oesen S, et al. Effects of elastic band resistance training and nutritional supplementation on muscle quality and circulating muscle growth and degradation factors of institutionalized elderly women: the Vienna Active Ageing Study (VAAS)[J]. Eur J Appl Physiol, 2016, 116(5): 885-897. DOI:10.1007/s00421-016-3344-8 |
| [17] | Bloch SAA, Lee JY, Syburra T, et al. Increased expression of GDF-15 may mediate ICU-acquired weakness by down-regulating muscle microRNAs[J]. Thorax, 2015, 70(3): 219-228. DOI:10.1136/thoraxjnl-2014-206225 |
| [18] | Minegishi Y, Inoue S, Sato K, et al. Smaller erector spinae muscle size is associated with inability to recover activities of daily living after pneumonia treatment[J]. Respir Investig, 2019, 57(2): 191-197. DOI:10.1016/j.resinv.2018.11.002 |
| [19] | Moon SW, Choi JS, Lee SH, et al. Thoracic skeletal muscle quantification: low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients[J]. Respir Res, 2019, 20: 35. DOI:10.1186/s12931-019-1001-6 |
| [20] | Verceles AC, Wells CL, Sorkin JD, et al. A multimodal rehabilitation program for patients with ICU acquired weakness improves ventilator weaning and discharge home[J]. J Crit Care, 2018, 47: 204-210. DOI:10.1016/j.jcrc.2018.07.006 |
| [21] | Hermans G, van den Berghe G. Clinical review: intensive care unit acquired weakness[J]. Crit Care, 2015, 19: 274. DOI:10.1186/s13054-015-0993-7 |
 2020, Vol. 29
2020, Vol. 29